Thermodynamics and Statistical Mechanics
Update 02-03-21: rewritten parts and expanded. Thermodynamics started as the study of heat engines, but applies to all
processes in the universe, including those of chemistry and, crucially,
the workings of life at every level. So, though this theme concerns the
thermodynamics of living processes, we introduce it with a picture
(right) of
perhaps the
quintessence of thermodynamics: the formation and working of stars.
This after all is where the chemical elements - used by all known life
- are created,
along with the
source of energy for very nearly all of life on earth (we mean our
nearest
star, the Sun, of course).
Thermodynamics (literally heat movement) is
the study of the flows of energy, its properties and the consequences
of its flowing. We already know that all life needs a source of energy,
but do we really know why? The way to understand it is through understanding thermodynamics. It turns out to be a most
fundamental topic because literally nothing at all can happen,
anywhere, in any way, without the flow of energy.
Nobody
really knows what energy is (it remains a mystery). What we can say,
having done our thermodynamics, is that it is preserved
(effectively eternal), never created nor destroyed* and therefore never
consumed, or burned or made by other things in the universe, including
power stations, trees and hungry husky dogs. That is the first law of thermodynamics.
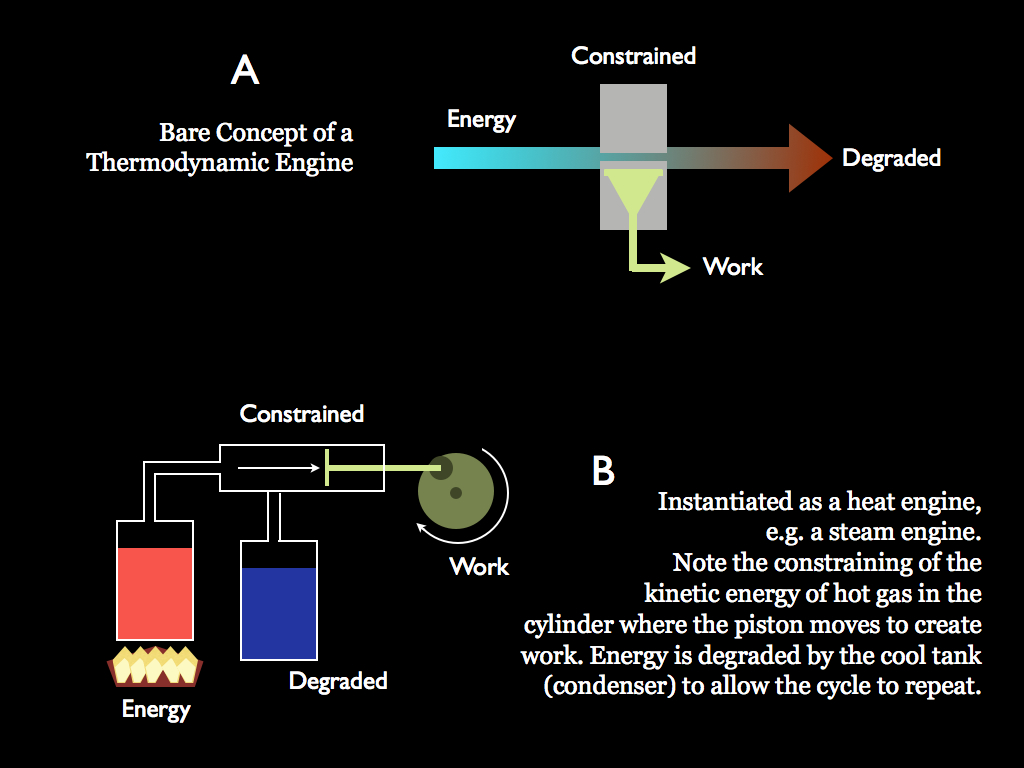
We abstract physical reality by conceptually isolating a part of it and saying that part has a quantifiable amount of internal energy (it might exchange energy with the rest of the universe, in which case we will call it open, otherwise it is a closed system). The internal energy of the system can always be partitioned into two categories - that which is available to do work and that which is not. This distinction is really one of whether energy can be constrained in its flowing or not. If it can be, then it is available to do work because work is the result of constraining flowing energy. When energy flows, two possible things can happen: a) the system might do something like move (or more elaborately get organised) or b) it may just dissipate. The latter (b) is the result of flowing energy that is not available to do work and the former (a) is work.
Another way to think of work is that it is the macroscopic effect of energy transfer, where macroscopic means anything on a scale larger than atoms. Heat is really just particles (atoms, molecules or plasma ions) moving at random, or vibrating, so if they are not in any way constrained, they just dissipate and nothing else happens at a physical scale greater than the particles themselves. Put them in a chamber and let them push against a movable wall (a piston) and they do work because at the larger (macroscopic) scale, they can move the piston. The chamber and movable wall are, of course, constraints on the flow of energy. This view is an example of statistical mechanics which is a molecular scale explanation for the classical phenomena of thermodynamics that were studied (mostly in the 18th century), especially those concerned with engines.
This matters for those interested in life for two reasons. Firstly, all life needs a source of energy because all life needs to work, literally, as an engine. Secondly, because it explicitly reveals a connection between energy, the work it does, and (weirdly for those who are new to this) information. The connection between these is the property of entropy - a much misunderstood (but not necessarily very hard) concept of great explanatory power. Here is a simple truth that will help a lot: constraint of energy flow is only possible if information is embodied by the system. The information embodied gives the system its form and this provides the constraint (as in the chamber and piston). The constraint of energy flow gives rise to work and one product of that work can be to embody information which then goes on to constrain more energy flow: a causal cycle is established.
The statistical mechanics
interpretation of
thermodynamics has both inspired and confused the use of information
theory in biology (and other fields beyond physics), most notably
regarding entropy.
Here we try to convey
a clear understanding of both
thermodynamics and statistical mechanics interpretations of living
processes in a way that supports the integrated description of life as
an information-process, at all levels from atoms to the global
ecosystem. Thermodynamics provides a conceptual link between energy,
information and the evolution of everything in our universe.
Some fundamental principles
All of living involves the transformation of energy from one form to another: in this respect life as a whole and all individual organisms themselves are engines. Since energy transformations can never be 100% efficient, all living things radiate heat energy (i.e they glow in the infra-red and indeed this has been proposes as one of the methods for detecting life beyond our planet). The essential reason why living must transform energy is that this is the way to 'pump' entropy* from with a system (the organism in this case) to the outside world. This is essential because to live, a system must maintain a high level of organisation and to do that requires work to be done. Thermodynamics teaches us that work can only be obtained by 'pumping' out entropy, usually into passing energy. As it gains the excess entropy, energy degrades from high value (such as light) to low value (usually heat). This is what engines do - they pump entropy from hot things into cold things: it was the study of steam engines that gave us these insights in the first place and from this developed the science of thermodynamics.
* Note that since entropy is a property of a system (describing its 'spread'), it is not a material, nor energy, it cannot really be 'pumped' - this is just an analogy using rather figurative language, as explained on our page about entropy. Technically, heat energy is being exported, which means that the proportion of total internal energy available for work (not heat) increases.This idea of entropy does not only apply to engines. In fact the
thermodynamic
interpretation is well established and very useful in chemistry and
therefore biochemistry, where it explains the necessary conditions and
direction of all of life’s chemical reactions, including, for example,
the
transcription of DNA. Indeed, thermodynamics is needed to understand
every level of the organisation of life, from chemical reactions all
the way up to the flows of energy and materials in ecological systems:
these flows all obey the laws of
thermodynamics, enabling us to quantify the processes that make up
ecological functions. Understanding this is critical to understanding
how life originated:
it must have been able to maintain a state of thermodynamic
non-equilibrium, meaning that thermodynamic gradients (differences)
were constantly maintained. They still are, of course. Living cells are
little engines
in the following sense: they transform energy from more to less
organised forms (increasing the dispersion of energy density, e.g. from
chemical bonds to heat) and do so in a way that performs work. In
thermodynamics, work is the organised (coherent) movement, or
combination (in the sense of making chemical bonds) of material, as
opposed to random movement and bond-making and breaking. This coherence
is a specific consequence of the organisational structure of the little
engine: it is the inevitable result of the information embodied in the
cell's chemical and physical structure. The extra problem for life, as
opposed to any human-made machine, is that life is solely responsible
for making, maintaining and reproducing itself: the process of
autopoiesis. It is the causal cycle referred to earlier that makes this possible.
The Theme is led by Dr Keith Farnsworth