Autocatalytic Sets
This page is still under constructionGroups of molecules (or other agents) that acting together make themselves, are obviously a good basis for autopoiesis and even autonomy. Here is a short introduction to them.
Catalysis usually refers to chemical reactions (though it is a common language concept): a catalyst is an agent (usually a substance) that accelerates a reaction, without itself being permanently transformed. To be general we can think of an inhibitor in the same way (slows the reaction down). 'Not permanently transformed' means that it takes part in the reaction but is unchanged by it, or if it undergoes a change it recovers very soon after with no loss of energy. In common language, we can be more general and say, for example, that alcohol is a catalyst of social interaction - or if you prefer, a shared meal is - though in both those cases, you will note the transformation of the putative catalyst is permanent (glug), so it's not strictly a catalyst at all. Probably the best known catalytic process these days is what the 'cat' does on your car exhaust (it's a catalytic converter, of course). A good example from biology is so good, it is actually called 'catalase': this is an enzyme that catalyses the break-down of H2O2 (hydrogen peroxide), which is a rather destructive substance produced as an unwanted by-product of several cellular processes: the cell needs to get rid of it quickly. So quickly does it work, that the bombadier beetle has harnested its power to blast a jet of steam out of its back end - an effective weapon indeed.
As you might expect, an autocatalytic process is one that catalyses itself, more precisely a reaction that is catalysed by one of its products. This can of course lead to a run-away reaction and in a sense, fire is the best known example, since fire is the exothermic oxygenation of 'fuel' that needs heat to run, but being exothermic, it produces heat and so once it has started... well you know what happens. Something similar, but far more dramatic is the nuclear fission chain-reaction. Going off like a firework rather sums up autocatalytic reactions and that is because, by definition, they form a short positive-feedback loop: they reinforce themselves.
It is only when we get more complicated that autocatalytic processes can be controllable, well-manered and even orderly (especially, of course, if we include the possibility of inhibitors).
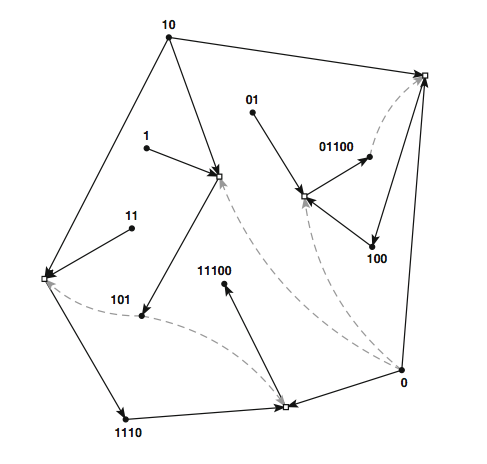
An example of Stuart Kauffman's binary autocatalytic set idea (diagram
taken from Hordijk et al. 2012*). 'Molecules' are represented as binary
strings. Reactions are either ligations (joining together, e.g. 11 +
101 -> 11101) or cleavings (cutting: 11011->11 + 011, though I
cannot see any examples of that in the diagram). Black dots here
represent the molecules (labelled), which enter reactions (denoted by
white boxes), to produce products (follow the arrows). The reactions
are catalysed by molecules - their contribution to the reaction being
denoted by the grey dotted arrows. So, for example, 10 and 1
react under catalysis from 0 to make 101 (a ligation) which then
becomes the catalyst for ligating 10 and 11 to make 1110 and also for
the ligation of 1110 and 0 to make 11100. There is nothing here making
0 or 1 or 11: these are termed a 'food set' by some, or merely
substrait or raw materials by others.
* I think this, or something very like it also appears in at least one of Kauffman's books.
* I think this, or something very like it also appears in at least one of Kauffman's books.
A more formal definition
Hordijk and Steel (2004) and Hordijk et al. (2010) define their chemical reaction system by a tuple
Q = {X, R, C}, in which X is a set of molecular types, R a set of reactions and C a set of catalytic relations specifying which molecular types catalyse each member of R. The system is also provided with a set of resource molecules F ⊆ X, freely available in the environment, to serve as raw materials for anabolism (noting that whilst we are defining an organisational closure, we may (and indeed must) permit the system to be materially and thermodynamically open).
The autocatalytic set is that subset of reactions R′⊆ R, strictly involving the subset X′⊆ X, which is:
• reflexively autocatalytic:
every reaction r ∈ R′ is catalysed by at least one of molecular type x′∈ X′
• and composed of F by R':
all members of X' are created by the actions of R' on F ∪ X'.
This definition of an autocatalytic set is an application of the broader mathematical concept of closure and more specifically of transitive closure of a set, since when the autocatalytic set is represented as a network (of reactions), this network has the properties of transitive closure.
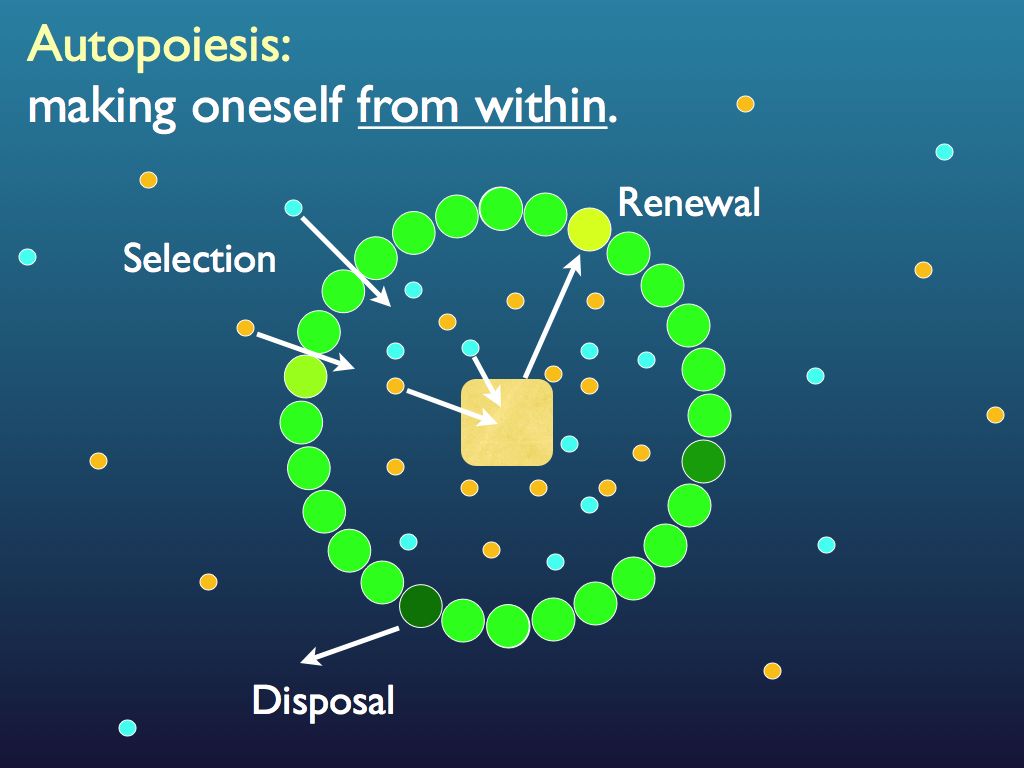
The concept of autocatalytic set has been implemented in experiments for exploring aspects of the origin of life (e.g., the GARD system simulating “lipid world” [Segré et al. 2000]). Clearly with the two conditions for an autocatalytic set met, everything in the system is made by the system, but there is a more important consequence. The system is made from the parts (only) and can only exist if they do. Organisational closure of this kind has been identified as a general property of individual organisms [37], many biochemical sub-systems of life [21] and embryonic development [38].
The Graded Autocatalysis Replication Domain (GARD).
One of the main theories about the origin of life starts with simple self-assembling membrane bags referred to as vesicles. Because the membranes are made from lipid rich molecules, this has won the nickname of "lipid world" (Segré et al, 2001).
In 2000, Doran Lancet and colleagues published a mathematical model of proto-life in a liposome (pre-biotic lipid-using vessicle) which they termed GARD. This is a model example of the class of origin of life hypotheses called 'lipid world' in which it is thought that life emerged from proto-cells that began as simple micelles or vesicles: lipid-based membrane 'bags' containing some substrate from which the parts of the membrane could be made (using resources that enter through the membrane). Structures like this abound in the real world.
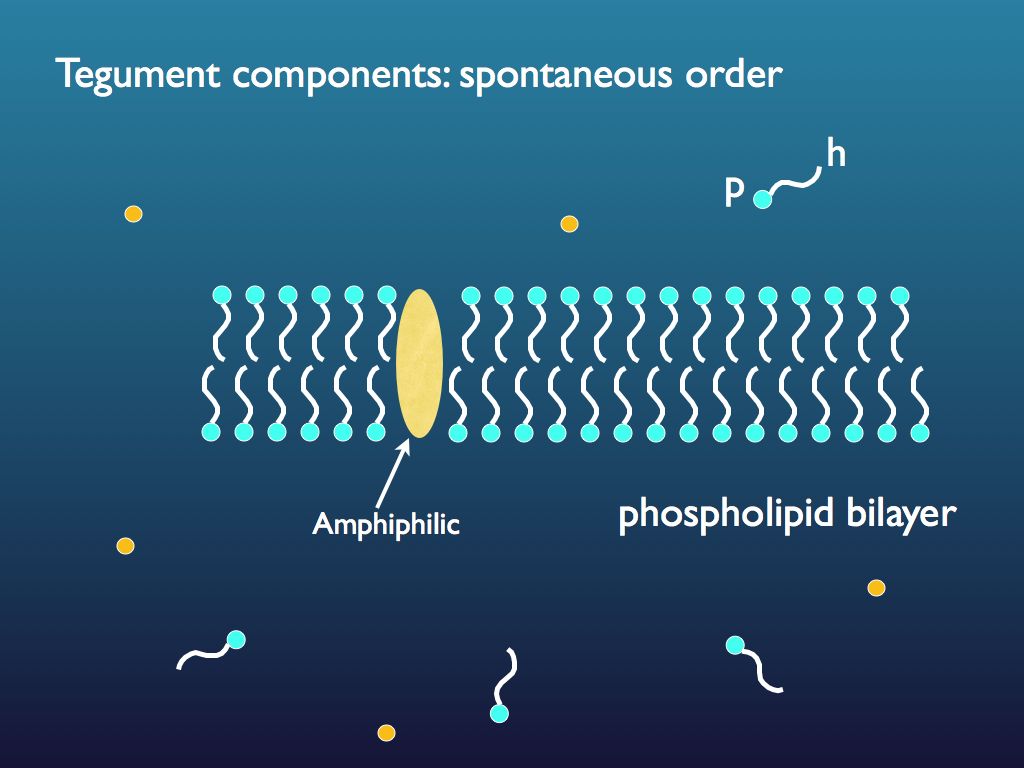
Luisi's vesicles
A common biological example of a lipid-based tegument is the phospholipid bilayer illustrated above. This is an important example of the general class of amphiphilic molecular assemblies that constitute membranes (amphiphilic means both hydrophilic and hydrophobic together - the little 'tadpole' molecules shown regimentally lined up to form the membrane sheet each have a hydrophilic end - the phosphorous P and a hydrophobic end - the hydrogen rich lipid tail h). In reasonable conditions (aqueous suspension), a population of amphiphilic molecules spontaneously self-assembles to form the membrane and when this happens different shapes form, among which little spherical bladders are very common - these are the vesicles. If a vesicle is able to continue growing, it will eventually undergo binary fission (just through mechanical instability) and so little vesicles of this kind perform a basic sort of population growth and are, in a very simple way, autopoietic. It is thought by some that things like this could have been the pre-cursor to life. This chemical system is a simple example of the 'lipid world' hypothesis of origin of life). [My diagrams were based on those appearing in Pier Louisi's book: Luisi, P. L. (2006).].
Here is a real-life example of a vesicle that was made in the laboratory:
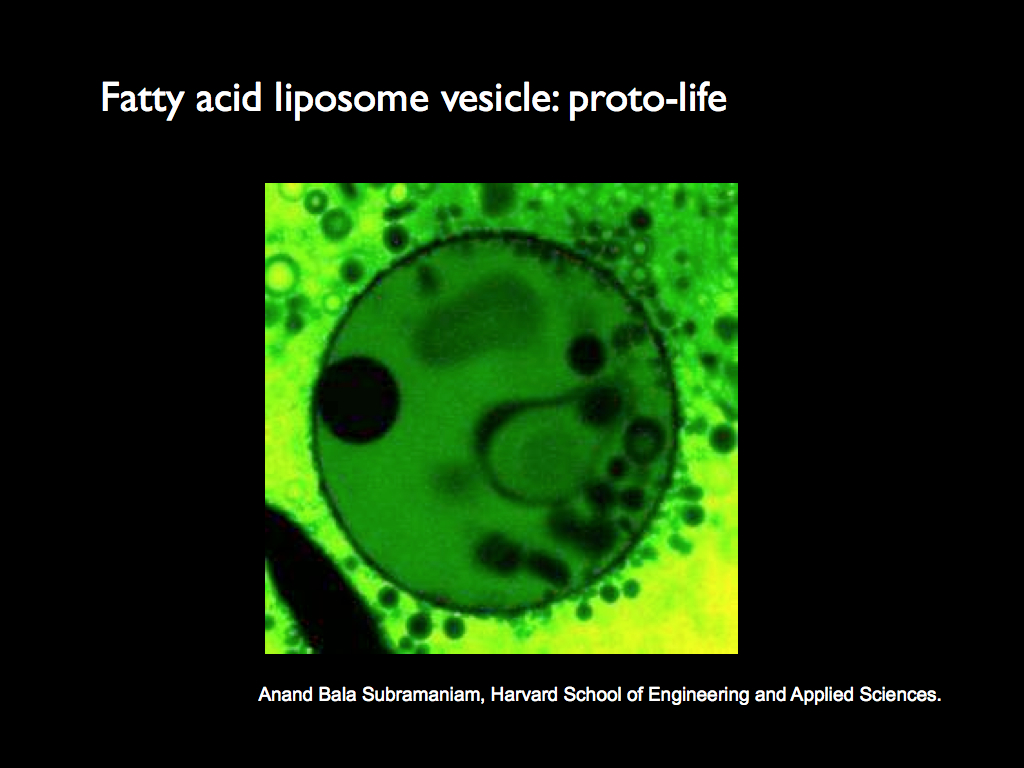
This image (and the liposome) were created by Anand Bala Subramaniam, Harvard School of Engineering and Applied Sciences (the term 'liposome' is often used to mean a vesicle made from lipid-based molecules, though strictly speaking they are not purely lipid, so should not really be referred to as that).
A crucial result of an amphiphilic vessicle is that the enclosed interior can have a different composition (e.g. concentration of chemicals) from the exterior environment. If the interior composition can be controlled, then there is the possibility of life.
Relation with Organisational Closure and Autopoeisis
- note that 'closure' is explained in a box on the autonomy page.
A system with organisational closure is one in which the form of the whole is produced by the interactions among its component parts alone, without any exogenous intervention, i.e. it is self-organising and the usual example is a vortex (e.g. a whirlpool or cyclone). Systems with organisational closure are self-sustaining organisations (the organisation is information embodied as a transcendent complex) that only need an appropriate source of energy and raw materials to maintain their form. Taking a step closer to life, in an autocatalytic setof chemical reagents the members of the set operate on one another to produce one another and thereby also to produce the system of interactions that perform the autocatalytic operations. This is clearly important in establishing autonomy, though an autocatalytic set alone cannot sustain itself without also involving a containment boundary. The most practical reason is that without a boundary, the component molecules would disperse by diffusion and reach a point where they just would not meet each other enough to sustain the chemistry (death by dliution). A system which creates its own transcendent complex, and also all the component parts from which it itself is made, and can sustain itself, is an autopoietic system, though this does not imply the atoms themselves are made by it (normally it means simpler -- raw material -- molecules are selected from the environment and assembled into functional molecular components). So, by no means all autocatalytic sets are autopoietic, but all autopoietic systems must operate as an autocatalytic set, among other things. Organisational closure is buit into the definition of the autocatalytic set (see formal definition above), but is also ensured by the operation of an autocatalytic set. That is all cybernetics, though. In material terms, it is essential for autopoiesis that the system materially makes itself, and of course this is also ensured by a chemical autocatalytic set.
References
Bitbol, M. and Luisi, P. (2004). Autopoiesis with or without cognition: defining life at its edge. J Royal Soc Interface, 1(1):99–107.
Hordijk, W.; Steel, M. (2004). Detecting autocatalytic, self-sustaining sets in chemical reaction systems. J. Theor. Biol. 227, 451–461.
Hordijk, W.; Hein, J.; Steel, M. (2010). Autocatalytic Sets and the Origin of Life. Entropy 12, 1733–1742
Hordijk, W., Steel, M., Kauffman, S. (2012). The Structure of Autocatalytic Sets: Evolvability, Enablement, and Emergence. Acta Biotheoretical 60:379–392 DOI 10.1007/s10441-012-9165-1
Luisi, P.-L. (2006). The Emergence of Life: From Chemical Origins to Synthetic Biology. Cambridge: Cambridge University Press.
Segré, D., Ben-Eli, D., Deamer, D. W., Lancet, D. (2001). The Lipid World. Origins of Life and Evolution of the Biosphere 31: 119–145
Segré, D., Ben-Eli, D., Lancet, D. (2000). Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. 97, 4112–4117.